6th Grade Unit 1: Energy Roadmap
After Unit 0: Groupwork, the 6th Grade science curriculum consists of 4 Units with 3 subunits.
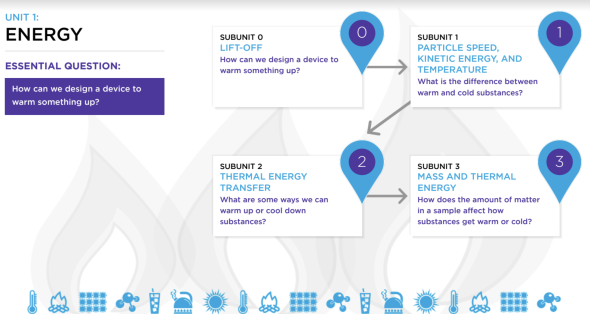
This page hosts Unit 1: Energy with the 3 subunits within the unit.
Subunit 1: Particle Speed, Kinetic Energy & Temperature
Link to this section
Below you will view and download:
🟧 Subunit Assessment Opportunities
🟧 5E Lesson Sequence
Subunit 1: Assessment Opportunities
Subunit 1 Assessment Opportunities
What should my students know and be able to do?
What should I prioritize?
Note: The materials below are personal recommendations from teachers in the field.
Feel free to consider your context when deciding whether to follow these suggestions.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
View and download (by making a copy)- Subunit 1 Assessments
Subunit 1: 5E Lesson Sequence
Subunit Description
📂 Download ALL lessons at one time for Unit 1: Subunit 1 from this folder. 📂
In this subunit, students consider substances at two scales: the macroscopic (observable) and particle scales. Through demonstrations and experiments, students observe the effects of adding or removing energy at the macroscopic scale, which includes temperature and phase changes, and particle scale, which includes changes in particles’ motion and kinetic energy. The main learning goal of this unit is for students to construct an understanding of the relationship between thermal energy, particle movement, kinetic energy, and temperature. We use the Subunit Essential Question to drive the learning for this subunit. See Assessment Tab for Sample Student Response.
The Big Conceptual Goals for this 5E cycle are
- Hot and cold are our experiences of what is going on at the particle scale of matter.
- When we add or remove energy from a substance, the particle motion and temperature change.
| Lesson | Lesson Name | Teacher Document | Student Handout |
|---|---|---|---|
| 1 | Engage |
|
|
| 2 | Explore | ||
| 3 | Explain | ||
| 4 | Elaborate | 6.1 SU1 4Elaborate Teacher | 6.1 SU1 4Elaborate Student |
| 5 | Evaluate | 6.1 SU1 5Evaluate Student | |
📂 Download ALL lessons at one time for Unit 1: Subunit 1 from this folder. 📂
Subunit 2: Thermal Energy Transfer
Link to this section
Below you will view and download:
🟧 Subunit Assessment Opportunities
🟧 5E Lesson Sequence
Subunit 2: Assessment Opportunities
Subunit 2 Assessment Opportunities
What should my students know and be able to do?
What should I prioritize?
Note: The materials below are personal recommendations from teachers in the field.
Feel free to consider your context when deciding whether to follow these suggestions.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
View and download (by making a copy)- Subunit 2 Assessments
Subunit 2: 5E Lesson Sequence
Subunit Description
📂 Download ALL lessons at one time for Unit 1: Subunit 2 from this folder. 📂
In this subunit, students consider how objects warm up or cool down. Using evidence from investigations, students notice that energy can transfer between objects that are touching or between objects that are not touching. Students also discover that energy is transferred from objects that are hotter to objects that are colder. Students read an article that tells them about how these processes translate to the particle scale and construct models that can be applied to their Culminating Project.
The Big Conceptual Goals for this 5E cycle are
- Particles moving in a substance collide with other particles to transfer thermal energy from the particles with higher average kinetic energy to the particles with lower kinetic energy (warmer to cooler).
- A substance can absorb thermal energy from electromagnetic waves without touching the radiating source.
- Certain materials maximize thermal energy transfer through conduction, while other materials maximize thermal energy transfer through radiation.
| Lesson | Lesson Name | Teacher Document | Student Handout |
|---|---|---|---|
| 1 | Engage | ||
| 2 | Explore | ||
| 3 | Explain | ||
| 4 | Elaborate | 6.1 SU2 4Elaborate Teacher | 6.1 SU2 4Elaborate Student |
| 5 | Evaluate | 6.1 SU2 5Evaluate Teacher | 6.1 SU2 5Evaluate Student |
📂 Download ALL lessons at one time for Unit 1: Subunit 2 from this folder. 📂
Subunit 3: Mass and Thermal Energy
Link to this section
Below you will view and download:
🟧 Subunit Assessment Opportunities
🟧 5E Lesson Sequence
Subunit 3: Assessment Opportunities
Subunit 3 Assessment Opportunties
What should my students know and be able to do?
What should I prioritize?
Note: The materials below are personal recommendations from teachers in the field.
Feel free to consider your context when deciding whether to follow these suggestions.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Subunit 3: 5E Lesson Sequence
Subunit Description
📂 Download ALL lessons at one time for Unit 1: Subunit 3 from this folder. 📂
Through several demonstrations and designing their own experiments, students learn that the mass of a sample plays a role in how easy or difficult it is to raise the temperature of a substance. Students apply this understanding to the Culminating Project and then design, construct, test, and modify a device that can maximize energy transfer to cookies or water in a hot tub.
The Big Conceptual Goals for this 5E cycle are
- A sample with more mass must absorb more thermal radiation in order to raise the average kinetic energy of all the particles in the sample, making it more difficult to heat a sample with more mass.
- In order to meet the criteria of a design problem, we need to take into account both scientific principles AND the potential impacts on people.
- A solution needs to be tested, and then modified on the basis of the test results, in order to be improved.
| Lesson | Lesson Name | Teacher Document | Student Handout |
|---|---|---|---|
| 1 | Engage |
|
|
| 2 | Explore | ||
| 3 | Explain | ||
| 4 | Elaborate | 6.1 SU3 4Elaborate Teacher | 6.1 SU3 4Elaborate Student |
| 5 | Evaluate |
6.1 SU3 Individual Patent Application Template
|
|
📂 Download ALL lessons at one time for Unit 1: Subunit 3 from this folder. 📂
Unit 1: Energy Documents
Link to this section
Below you will view and download: Unit Plan, Standards, Culminating Project Assessments and Rubrics, Common Misconceptions, Materials, Unit 0: Lift-Off Lessons and Resources.
6.1 Energy: Overview
Overview
Through investigations in the Energy Unit, students will be able to identify the relationships between thermal energy transfer, particle speed, kinetic energy, and temperature—including identifying where thermal energy is transferred from and to. They will also be able to distinguish between thermal energy and the temperature of substances and describe the relationships among mass, type of substance, and change in temperature. For the Culminating Project, students will engineer a device that maximizes thermal energy transfer into a system to meet the needs of one of two possible clients. Students will work collaboratively to plan, build, test, and revise their client’s device. Each student will produce an Individual Patent Application to describe the processes of designing, constructing, testing, and revising their device.
6.1 Energy: Unit Plan
Unit 1: Energy - Unit Plan
|
|
||
|
|
||
|
|
|
|
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
||||
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
“Disciplinary Core Ideas, Science and Engineering Practices, and Crosscutting Concepts” are reproduced verbatim from A Framework for K-12 Science Education: Practices, Crosscutting Concepts, and Core Ideas. DOI: https://doi.org/10.17226/13165. National Research Council; Division of Behavioral and Social Sciences and Education; Board on Science Education; Committee on a Conceptual Framework for New K-12 Science Education Standards. National Academies Press, Washington, DC. This material may be reproduced for noncommercial purposes and used by other parties with this attribution. If the original material is altered in any way, the attribution must state that the material is adapted from the original. All other rights reserved.
6.1 Energy: Standards
Human Impact on Earth’s Climate
Next Generation Science Standards Performance Expectations
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NGSS Lead States. 2013. Next Generation Science Standards: For States, By States. Washington, DC: The National Academies Press.
*This performance expectation integrates traditional science content with engineering through a Practice or Disciplinary Core Idea.
Disciplinary Core Ideas
PS3.A: Definitions of Energy
- The term “heat” as used in everyday language refers both to thermal energy (the motion of atoms or molecules within a substance) and the transfer of that thermal energy from one object to another. In science, heat is used only for this second meaning; it refers to the energy transferred due to the temperature difference between two objects.
- Temperature is a measure of the average kinetic energy of particles of matter. The relationship between the temperature and the total energy of a system depends on the types, states, and amounts of matter present.
- Temperature is not a measure of energy; the relationship between the temperature and the total energy of a system depends on the types, states, and amounts of matter present.
- The temperature of a system is proportional to the average internal kinetic energy and potential energy per atom or molecule (whichever is the appropriate building block for the system’s material). The details of that relationship depend on the type of atom or molecule and the interactions among the atoms in the material. Temperature is not a direct measure of a system’s total thermal energy. The total thermal energy (sometimes called the total internal energy) of a system depends jointly on the temperature, the total number of atoms in the system, and the state of the material.
PS3.B: Conservation of Energy and Energy Transfer
- The amount of energy transfer needed to change the temperature of a matter sample by a given amount depends on the nature of the matter, the size of the sample, and the environment.
- Energy is spontaneously transferred out of hotter regions or objects and into colder ones.
ETS1.A: Defining and Delimiting an Engineering Problem
- The more precisely a design task’s criteria and constraints can be defined, the more likely it is that the designed solution will be successful. Specification of constraints includes consideration of scientific principles and other relevant knowledge that is likely to limit possible solutions.
ETS1.B: Developing Possible Solutions
- A solution needs to be tested and then modified on the basis of the test results in order to improve it. There are systematic processes for evaluating solutions with respect to how well they meet criteria and constraints of a problem.
- Models of all kinds are important for testing solutions.
ETS1.C Optimizing the Design Solution
- The iterative process of testing the most promising solutions and modifying what is proposed on the basis of the test results leads to greater refinement and ultimately to an optimal solution.
Science and Engineering Practices
Asking Questions and Defining Problems (in the Lift-Off)
- Ask questions that arise from careful observation of phenomena, models, or unexpected results, to clarify and/or seek additional information.
- Ask questions to clarify and/or refine a model, an explanation, or an engineering problem.
*Developing and Using Models (Focal Practice)
- Develop a model to predict and/or describe phenomena.
- Develop and/or use a model to generate data to test ideas about phenomena in natural or designed systems, including those representing inputs and outputs, and those at unobservable scales.
- Develop a model to describe unobservable mechanisms.
Planning and Carrying Out Investigations
- Plan an investigation individually and collaboratively and, in the design, identify independent and dependent variables and controls, the tools needed to gather the data, how measurements will be recorded, and how much data are needed to support a claim.
- Collect data to produce data to serve as the basis for evidence to answer scientific questions or test design solutions under a range of conditions.
Analyzing and Interpreting Data
- Analyze and interpret data to determine similarities and differences in findings.
Constructing Explanations and Designing Solutions
- Apply scientific ideas or principles to design, construct, and/or test the design of an object, tool, process or system.
- Construct an explanation using models or representations.
Engaging in Argument from Evidence
- Construct, use, and/or present an oral and written argument supported by empirical evidence and scientific reasoning to support or refute an explanation or a model for a phenomenon or a solution to a problem.
- Respectfully provide and receive critiques about one’s explanations, procedures, models, and questions by citing relevant evidence and posing and responding to questions that elicit pertinent elaboration and detail.
Obtaining, Evaluating, and Communicating Information
- Critically read scientific texts adapted for classroom use to determine the central ideas and/or obtain scientific and/or technical information to describe patterns in and/or evidence about the natural and designed world(s).
- Communicate scientific and/or technical information (e.g., about a proposed object, tool, process, system) in writing and/or through oral presentations.
Crosscutting Concepts
Cause and Effect
- Cause and effect relationships may be used to predict phenomena in natural or designed systems.
Scale, Proportion, and Quantity
- Time, space, and energy phenomena can be observed at various scales using models to study systems that are too large or too small.
- Phenomena that can be observed at one scale may not be observable at another scale.
Systems and System Models
- Models can be used to represent systems and their interactions—such as inputs, processes, and outputs—and energy and matter flows within systems.
*Energy and Matter (Focal Crosscutting Concept)
- The transfer of energy can be tracked as energy flows through a designed or natural system.
- Energy may take different forms (e.g., energy in fields, thermal energy, energy of motion).
“Disciplinary Core Ideas, Science and Engineering Practices, and Crosscutting Concepts” are reproduced verbatim from A Framework for K-12 Science Education: Practices, Crosscutting Concepts, and Core Ideas. DOI: https://doi.org/10.17226/13165. National Research Council; Division of Behavioral and Social Sciences and Education; Board on Science Education; Committee on a Conceptual Framework for New K-12 Science Education Standards. National Academies Press, Washington, DC. This material may be reproduced for noncommercial purposes and used by other parties with this attribution. If the original material is altered in any way, the attribution must state that the material is adapted from the original. All other rights reserved.
Connections to Nature of Science
Scientific Knowledge Is Based on Empirical Evidence
- Science knowledge is based upon logical and conceptual connections between evidence and explanations.
NGSS Lead States. 2013. Next Generation Science Standards: For States, By States. Washington, DC: The National Academies Press.
Link to Connect the 6th Grade Energy Unit with Prior Knowledge.
6.1 Energy: Culminating Project Assessments and Rubrics
Culminating Project Assessments and Rubrics
📂Download ALL files from 6.1 Culminating Project Assessments folder.📂
| Culminating Project File Docs |
|---|
| 6.1 Main–Culminating Projects |
| 6.1 Oral Presentation Rubric |
| 6.1 Science and Engineering Content Rubric |
| 6.1 Science and Engineering Practices Rubric |
📂Download ALL files from 6.1 Culminating Project Assessments folder.📂
6.1 Energy: Common Misconceptions
Common Misconceptions
Lift-Off
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Subunit 1: Particle Speed, Kinetic Energy, and Temperature
|
|
|
|
|
|
|
|
(http://assessment.aaas.org/misconceptions/1/AM/29/AMM107)http://assessment.aaas.org/misconceptions/1/AM/29/AMM009) |
Subunit 2: Thermal Energy Transfer
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Subunit 3: Mass and Thermal Energy
6.1 Energy: Materials
Materials
View and download (by making a copy) of Materials
The Unit 1: Energy Materials table includes all of the items needed to teach five sections of this unit in a classroom of 32 students (eight groups of four). A detailed breakdown of how these items are used throughout the unit can be found in your Teacher Background Section at the subunit level and in each individual lesson in your Teacher Edition.
- Permanent materials have already been provided to all middle schools in the district and are expected to be reused from year to year.
- Consumable materials are replenished on an as-needed basis from year to year.
- Teacher Provided materials must be supplied by teachers each year.
Unit 1: Energy Materials
6.1 Energy: Subunit 0: Lift-Off Lessons
Subunit 0: Lift-Off
📂 Download ALL lessons at one time for Subunit 0: Lift-Off from this folder.📂
Lessons
| Lift-Off Lesson Documents |
|---|
| 6.1 SU0 General Groupwork Slide |
| 6.1 SU0 Liftoff Slides |
| 6.1 SU0 Liftoff Teacher |
| 6.1 SU0 Liftoff Student |
📂 Download ALL lessons at one time for Subunit 0: Lift-Off from this folder.📂
6.1 Energy: Want to know more about this unit?
Want to know more about this unit?
View and download (by making a copy) of Resources
Resources
Here are some resources for Unit 6.1 Energy:
Thermal Energy
Energy Foundations for High School Chemistry
“ENERGY FOUNDATIONS for High School Chemistry.” Energy Foundations for High School Chemistry. Accessed November 1, 2019. http://highschoolenergy.acs.org/content/hsef/en.html.
(These resources were written with the high school teacher in mind, but they could be helpful for those wanting to brush up on their understanding of thermal energy.)
Engineering Design
PBS LearningMedia California
“What Is the Engineering Design Process?” PBS LearningMedia. Building Big, October 4, 2019. https://ca.pbslearningmedia.org/resource/phy03.sci.engin.design.desprocess/what-is-the-engineering-design-process/#.XbMGDUX0k62.
(These resources are helpful for familiarizing yourself with the engineering design process students use during the unit.)
Experimental Design
National Center for Education Statistics: Kids’ Zone
“National Center for Education Statistics (NCES) Kids' Zone Home Page, Part of the U.S. Department of Education.” NCES Kids' Zone Test Your Knowledge. Accessed November 1, 2019. https://nces.ed.gov/nceskids/createagraph/
Recommended Videos
BrainPOP: Scientific Method
“Scientific Method.” BrainPOP. Accessed November 1, 2019. https://www.brainpop.com/science/scientificinquiry/scientificmethod/.
Recommended Reading
Science Buddies: Variables in Your Science Fair Project
Science Buddies. “Variables in Your Science Fair Project.” Science Buddies. Science Buddies, August 28, 2019. https://www.sciencebuddies.org/science-fair-projects/science-fair/variables#whatarevariables.
mathxscience.com: Experimental Variables
“Middle School Science Help: Krystal Cortez: Scientific Method Variables.” Middle School Science Help | Krystal Cortez | Scientific Method Variables. Accessed November 1, 2019. http://mathxscience.com/scientific_method_variables.html.
Science Made Simple: Designing Science Fair Experiments
“Designing Science Fair Experiments by Science Made Simple.” Designing Science Fair Experiments. Accessed November 1, 2019. https://www.sciencemadesimple.com/science_fair_experiment.html.
The Human Spark: Experimenting with Experiments
“Experimenting with Experiments ~ Lesson Activities.” PBS. Public Broadcasting Service, January 19, 2011. https://www.pbs.org/wnet/humanspark/uncategorized/experimenting-with-experiments-lesson-activities/431/.
Assessment Practice Items
Stanford University: Stanford NGSS Assessment Project, Short-Response Items
“Short-Response Items.” Short-response items | Stanford NGSS Assessment Project. Accessed November 1, 2019. https://snapgse.stanford.edu/snap-assessments/short-response-items.
Other Resources Used in 6.1 Energy
“Beat the Heat!” NASA. NASA. Accessed November 1, 2019. https://spaceplace.nasa.gov/beat-the-heat/en/.
“Inverted Bottles.” Exploratorium, August 1, 2017. https://www.exploratorium.edu/snacks/inverted-bottles.
Lab Interactive. Accessed November 1, 2019. http://lab.concord.org/embeddable.html#interactives/sam/phase-change/6-phase-changes-caused-by-energy-input.json.
PhET Interactive Simulations, University of Colorado Boulder,
https://phet.colorado.edu.
“Observation & Inference.” Observation & Inference • Unit [T1] SciGen SERP. Accessed November 1, 2019.
https://serpmedia.org/scigen/t1.html.
View and download (by making a copy) of Resources
Note: The CC BY-NC 4.0 License does not apply to photos, images, articles, and other materials within the curriculum that have been licensed by San Francisco Unified School District and Stanford University (the Authors). These include but are not limited to photos from commercial stock photo/image agencies such as Shutterstock.com or Getty Images (iStock.com) and photos or graphics where the Authors obtained permission from organizations such as UCMP or SERP. This CC BY-NC 4.0 License also does not apply to articles that the Authors received permission to reprint [Reprinted with Permission]. You can identify such a photo, image, or licensed material by looking at the credit embedded within or associated with the content. You are allowed to reproduce the licensed material for your own personal, classroom, non-commercial use only, BUT (i) you may not modify, alter, adapt, or otherwise create any derivative work from, a licensed material and (ii) you may not distribute, transmit or disseminate a licensed material or any copy or derivative work thereof, to any third party, whether by itself, as part of a large works, or otherwise.
Note also, that throughout the student pages, there are some icons created by SFUSD and Stanford that may not have a credit line because of lack of space.
The culminating project icons that follow were created or photographed by the San Francisco Unified School District and Stanford University and are all [CC BY-NC 4.0]:
6th Grade Science Units Link to this section
This page was last updated on July 24, 2023







